The procedure to use the activation energy calculator is as follows. Y mx b.
How To Use An Arrhenius Plot To Calculate Activation Energy And Intercept Youtube
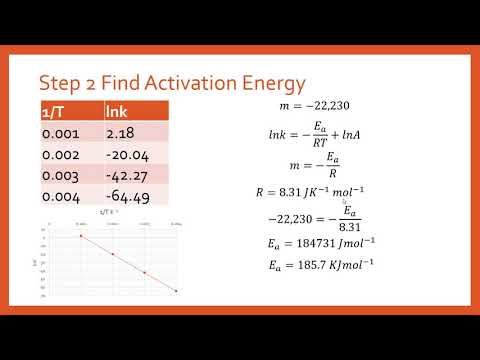
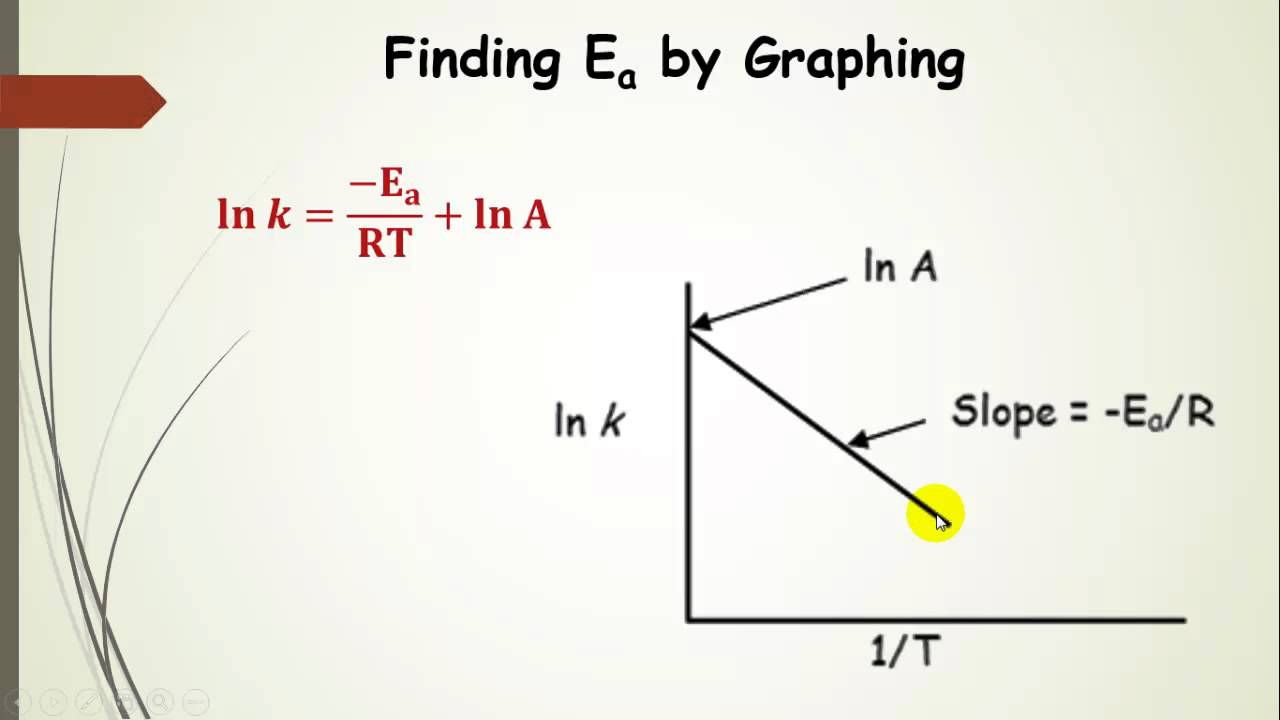
Another way to calculate the activation energy of a reaction is to graph ln k the rate constant versus 1T the inverse of the temperature in Kelvin.

. When the lnk rate constant is plotted versus the inverse of the temperature kelvin the slope is a straight line. However from my. M - E a R.
NOW Activation Energy. Science Chemistry QA Library Calculate the activation energy EA for the forward reaction. T 1 and T 2 absolute temperatures Kelvin k 1 and k 2 the reaction rate constants at T 1 and T 2.
Calculate the activation energy EA for the forward reaction. In this equation k is the rate constant for the reaction Z is a proportionality constant that varies from one reaction to another E a is the activation energy for the reaction R is the ideal gas constant in joules per mole kelvin and T is the temperature in kelvin. Now one use it to calculate the Activation Energy by making use of the graphing ink versus 1T.
Y is ln k x is 1T and m is -E a R. Taking the natural logarithm of both sides gives us. Now click the button Calculate Activation Energy to get the result.
R ideal gas constant83145 JKmol. The value of the slope m is equal to -EaR where R is a constant equal to 8314 Jmol-K. Formula to calculate activation energy.
In k frac-EaRleft frac1T right In A. Ie E a Threshold energy E Threshold - Average kinetic energy of the reacting molecules E. Ea activation energy of the reaction.
So now we can use it to calculate the Activation Energy by graphing lnk versus 1T. The Arrhenius equation can be used to determine the activation energy for a reaction. We start by taking the natural.
A slight rearrangement of this equation then gives us a straight line plot y mx b for ln k versus where the slope is. For example the Activation Energy for the forward reaction AB -- C D is 60 kJ and the Activation Energy for the reverse reaction C D -- A B is 80 kJ. NOW Activation Energy.
The activation energy for the reaction can be determined by finding the slope of the line. The Activated Complex is an unstable intermediate product that is formed during the reaction. The activation energy can be determined by finding the rate constant of a reaction at several different temperatures.
We can graphically determine the activation energy by manipulating the Arrhenius equation to put it into the form of a straight line. Once the reaction has obtained this amount of energy it must continue on. E a the activation energy of the reaction in Jmol.
Substracting equation 4 from equation 3 results in. The rate constant the activation energy and the Arrhenius parameter of a chemical reaction at 25C are 30xx10-4S-1 1044 KJ mol-1 asked 2 days ago in Chemistry by Sowaiba. The formula for calculating activation energy of diffusion.
The activation energy formula certainly is k Aefrac-EaRT Consider a situation where one rearranges this formula and takes the natural log of this particular. The question is like this. The activation energy can also be found algebraically by substituting two rate constants k 1 k 2 and the two corresponding reaction temperatures T 1 T 2 into the Arrhenius Equation 2.
Enter the temperature frequency factor rate constant in the input field. Activation Energy Graph - 18 images - reaction mechanism what happens to a molecule while it variation of activation energy with current download table media portfolio exergonic reaction definition equation graph and examples. The slope activation energy will be times - R 8314 JK1mol1 from -EaR slope.
Moreover one can put it into the format of a straight line. T1T2 Absolute Temperature in Kelvin. Or an intro to activation energy seehttpsyoutubehgQDMmzSOu4To solve for activation energy graphically seehttpsyoutubedrnYpMng90oIn this video we.
Q RT m K o v Where. To compute for activation energy of diffusion four essential parameters are needed and these parameters are Gas Constant R Melting Temperature of Metal T m Constant that depends on Metal Crystal Structure K o and Normal Valence in Metal v. So now we can use it to calculate the Activation Energy by graphing lnk versus 1T.
Calculate the activation energy EA for the forward reaction. When the lnk rate constant is plotted versus the inverse of the temperature kelvin the slope is a straight line. If we know the rate constant k1 and k2 at T1 and T2 the activation energy formula is.
From the literature that I have read the Ea of HDPE is around 150 - 300 kJmol. Calculate the activation energy needed to create a single vacancy in aluminium given. The plot will form a straight line expressed by the equation.
R the ideal gas constant 83145 JKmol. The activation energy is equal to the difference between the threshold energy needed for the reaction and the average kinetic energy of all the reacting molecules. The value of the slope m is equal to -EaR where R is a constant equal to 8314 Jmol-K.
Density 500 Celsius 262 grcm 3. Finally the activation energy required for the atoms or molecules will be displayed in the output field. Notice that when the Arrhenius equation is rearranged as above it is a linear equation with the form y mx b.
K1k2 the reaction rate constant at T1 and T2.
The Arrhenius Equation Activation Energy And Catalysis Explained Pt 8 Youtube

Activation Energy And Rate Constant For A Reversible Reaction Review Youtube

0 Comments